And now for an English version. There is something that bothers me: the science of medicinal research (in times of crisis).
In the Netherlands there was much ado about the increasing scarcity of lysis buffer, being an important solution used for the PCRs used for SARS-CoV-2 testing.
My first response to this was: ask a couple of chemists to make the stuff. There are plenty of recipes for this solution on the internet. Strange! Is this yet another example of examining challenges by a too narrow a group of domain experts?
A much more interesting question, when considering prevention and care, is the lack of possibilities to treat patients now. Of course, patients receive supportive care by means of e.g. ventilators in ICU’s, but drugs are not indicated and not used, as far as I know and is being communicated.
So that’s because there aren’t any drugs available for corona patients, right? Not quite. There are various agents we could use in care for these patients. And these belong in no way to the ‘alternative’ category.
Before discussing one of these agents that fits the bill for usage in SARS-COV-2 patients, I will discuss a ‘scientific entity’ that has, for decades, enjoyed way too much praise (to the detriment of patients and the medical field): the Randomised Control Trial aka RCT.
This refers especially to randomised studies with control groups. The prefered form of an RCT is the double-blind type -both researchers and patient are unaware whether the test drug (farmacon) or the placebo is administered- as this limits the influence of bias due to expectations.
The double blind RCT is usually referred to as the ‘golden standard’ of clinical trials. Only using this method determines if a drug ‘works’. Or rather, that is what is unjustifiably being taught in medical school and beyond. Let me explain.
(I will leave discussion on the uselessness of the p-value -‘significance’- for what it is. Please read the critiques of e.g. Matt Briggs and Frank Harrell.)
The basic premise of a RCT is simple enough: find the unique difference between two groups and elucidate the causal connection. As [John Stuart Mill]( “John Stuart Mill. 2019. Complete Works of John Stuart Mill. Delphi Classics.") told us:
“If an instance in which the phenomenon under investigation occurs, and an instance in which it does not occur, have every circumstance save one in common, that one occurring only in the former; the circumstance in which alone the two instances differ, is the effect, or cause, or a necessary part of the ca use, of the phenomenon.”
So, a RCT has two ‘wings’: a treament group, where the test drug is administered and a placebo group where all change in outcome is ‘natural’.
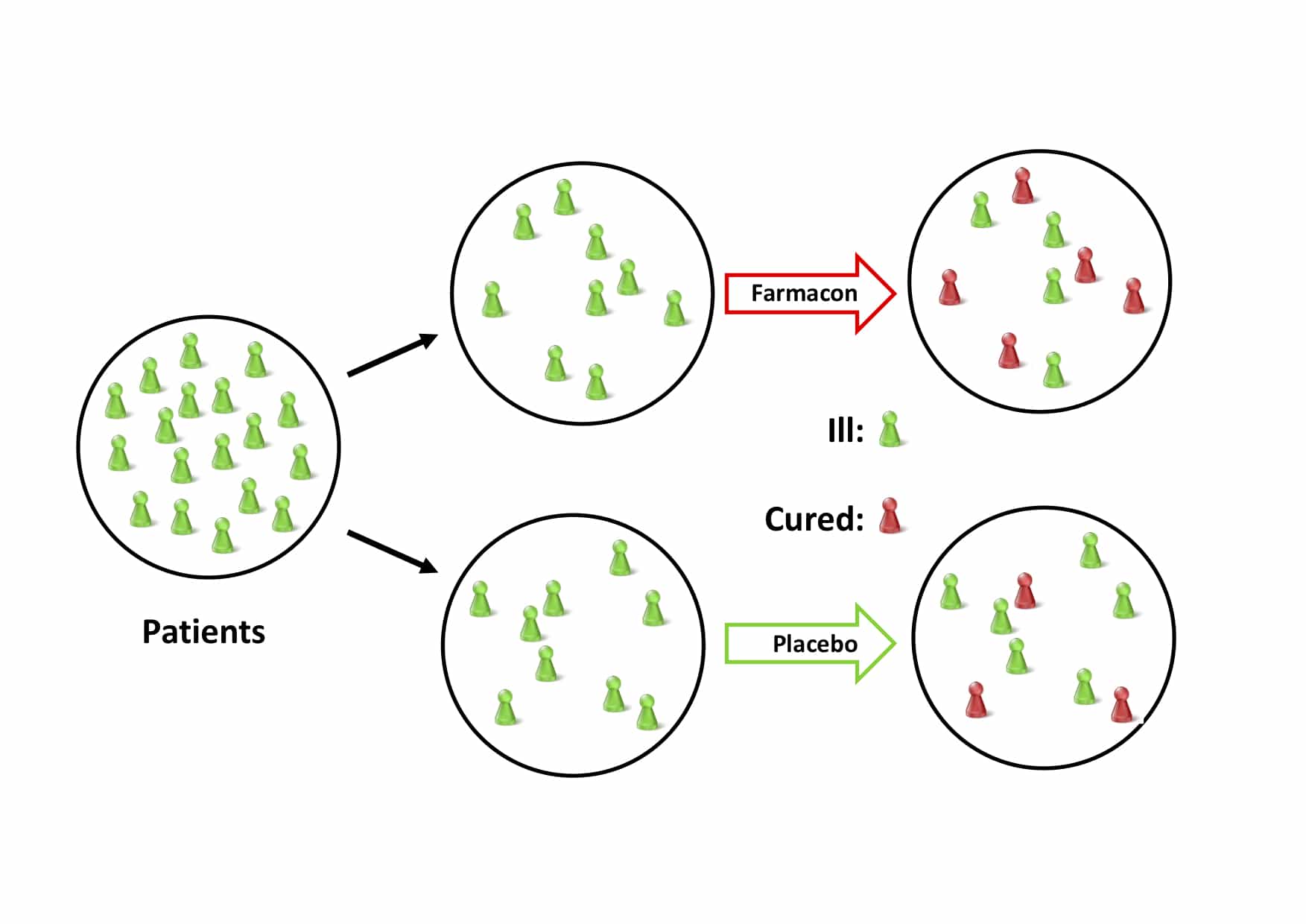
An ideal RCT clinches the result that the treatment/agent works somewhere. That is, if all requirements for an ideal study are met, a difference in outcome-probability between the treatment and control/placebo groups entails that the treatment caused the outcome, at least in some individuals in the population under scrutiny. Cartwright and Hardie [explain]( “Cartwright, N., Hardie, J. 2012. Evidence-Based Policy. A Practi- cal Guide to Doing It Better. Oxford University Press.") (italics added):
“The study design itself provides warrant for the assumptions required to underwrite the reliability of the results, without the need to import background causal knowledge from elsewhere. All the causes of the outcome other than the one under test are to be distributed in the same way in the treatment and control groups. But you do not need to know what these are in order to have some confidence that this requirement is satisfied. Masking, random assignment, and placebo control are supposed to satisfy this requirement for you.”
Unsurprisingly, there are problems with RCTs. One obvious problem is this: the circumstances of the RCTs are specifically designed for buttressing ‘it-works-somewhere’-claims (as Cartwright and Hardie so nicely explain), that is in some members of the trial. However, they do little if anything to establish the fact that the agent under scrutiny can play the same causal role elsewhere, that is ‘here’, in our situation.
This is complicated further by what Alexander Krauss calls the achieving-good-randomisation assumption. Participants, so the story goes for ideal RCTs, are randomised and equally distributed into trial groups along measurable, known ánd non-measurable and unknown background influencers. Kraus is not impressed (as we should all be):
“Poor randomisation – and thus poor distribution of participants’ background traits that affect outcomes between trial groups – puts into question the degree of robustness of the results from several of these 10 leading RCTs.”
Many confounding factors (background traits) that are either known or unknown and which are not equally distributed between the farmacon- and placebo group will cloud the outcome of the trial. Kraus depicts this lack of proper randomisations, as a result of which the placebo (control) and the farmacon (treatment) groups are not the same, as follows:
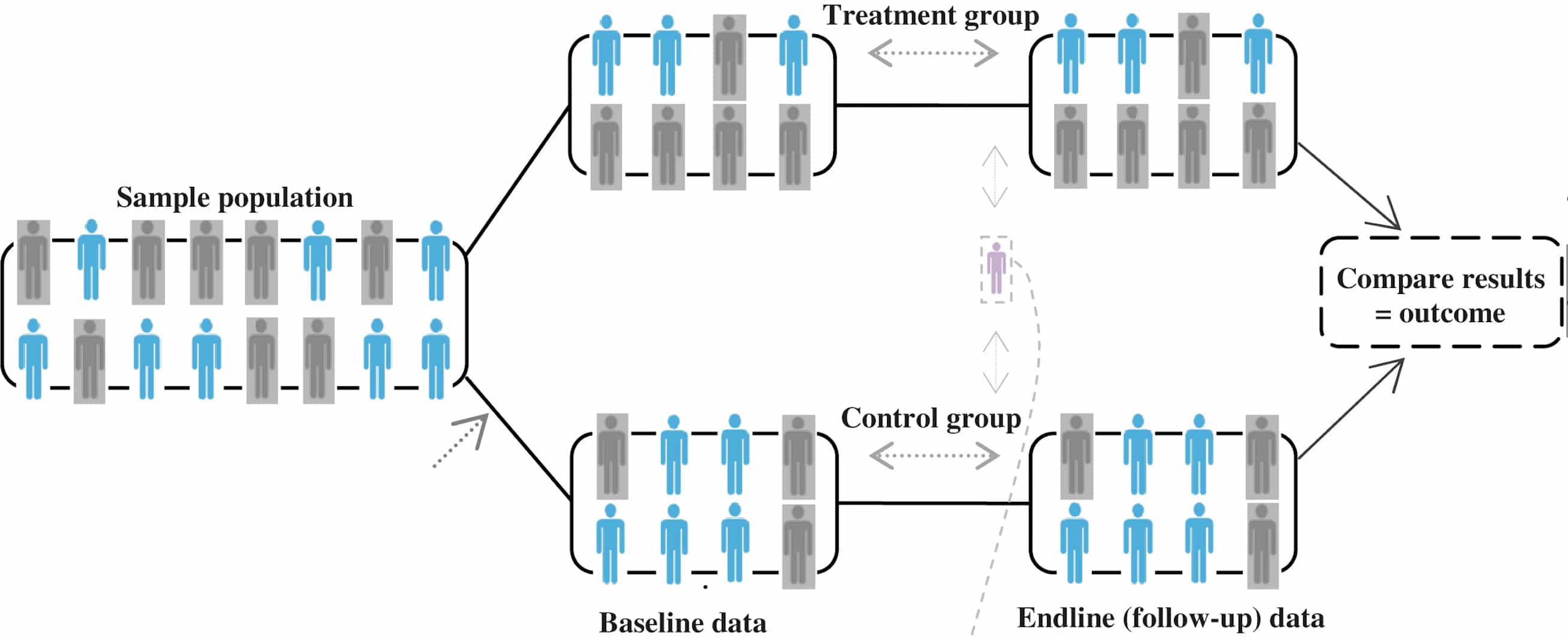
The little purple figurine in between the two groups depicts the participants who have missing data or switch between treatment and control groups, but also who do not comply or take full intended treatment, stop taking it, or drop out. All that will influence results as well, and not for the better.
Yet another problem lies in answering questions on how things work, and why, the quintessence of science. RCT are not in the business of elucidating mechanism and thus do not further any knowledge thereon. In fact, the medical ‘adoration’ of RCT might hamper mechanistic work.
So, when health organisations stress that certain chemicals should not be used because RCTs are lacking does not bode well for the authority of such organisations. They either have little idea what an RCT is and what its limitations are or just make a doctrinaire statements. (It’s the not-invented-here syndrome methodologically rationalised.)
This is far from an appeal to something like ‘anything goes’. Quite the contrary. It actually is an appeal to do the tough mechanistic work as standard; RCTs are no stand-ins for that.
And this brings me to the chemical that has been around for decades and on which quite a bit of (mechanistic and other) research has been spent and is still being done.
No, I am not talking about the antimalaria drugs chloroquine phosphate and hydroxychloroquine sulfate, although these are now authorised for emergency use by the FDA. This study gives some idea of the usefulness of these drugs.
I refer to N-Acetylcysteine (NAC), which is a precursor of glutathione, our own internal antioxidant defence molecule.
It is currently in stock in virtually all hospitals and emergency medical facilities throughout the world. It is on the list of World Health Organization Model List of Essential Medicines.
This [2019-book]( “Frye, R.E., Berk, M. 2019. The Therapeutic Use of N-Acetylcysteine (NAC) in Medicine. Springer Nature Singapore.") summarises the research being done on NAC, and it is rather impressive. NAC is an intermediate of cysteine metabolism and a popular prodrug used to rapidly ramp up cysteine levels in the body and thereby increasing our anti-oxidative protection. Vital in inflammatory situations such as viral infections.
NAC is a universally accepted antidote to acetaminophen (APAP; Tylenol, paracetamol) poisoning. It does its antidotal work via the glutathione route. It is also in use as a mucolytic drug, that is it aids in the clearance of mucus from the upper and lower airways.
Also, anti-viral research has been done on NAC, such as this 1997-study. This study concluded that NAC appears to provide an attenuation of influenza and influenza-like episodes, especially in elderly high-risk individuals. Much more research needs to be done on the anti-vital quality, if any, of NAC. Quite useful in times likes these.
Is it a silver/magic bullet agent? Of course not! Nothing ever is. Nevertheless, there is enough scientific evidence available to use it as a functional and safe agent.
Nevertheless, both the pharmaceutical and medical fields deem NAC hardly worth a look. I am guessing that this has to do with the fact that NAC is not patentable and is available at very low cost.
Nevertheless, it is odd that during this pandemic, no studies have been undertaken, as far as I know, to look closely at the qualities of NAC from a mechanistic point of view using biomarkers such as Interleukin-6, viral-load trends in patients treated with NAC, and recovery time of patients after hospitalisation. Other markers could be used as well.
In conclusion, it is time that the medical world should free itself from the methodological straitjacket which is RCT and think ‘outside that box’ to the benefit of many patients.
